Recalled Eye Drops List 2025. Lubricant eye drops 15 ml (single pack) carboxymethylcellulose sodium 0.5%. Select up & up eyedrops.
The fda has expanded its list of eye drops recalled in 2025 because the products could be tainted with bacteria. The fda has now officially recalled a slew of eye drop products, following an announcement from the facility that produces them, reports cbs news.
Full List Of Recalled Eye Drops Identify Potentially Contaminated, Full list of impacted products. The fda has expanded its list of eye drops recalled in 2025 because the products could be tainted with bacteria.

TopCare Eye Drops Recall, [1/31/2025] fda is warning consumers not to purchase or use south moon, rebright or fivfivgo eye drops because of the potential risk of eye infection. (wmt) and other brands has.
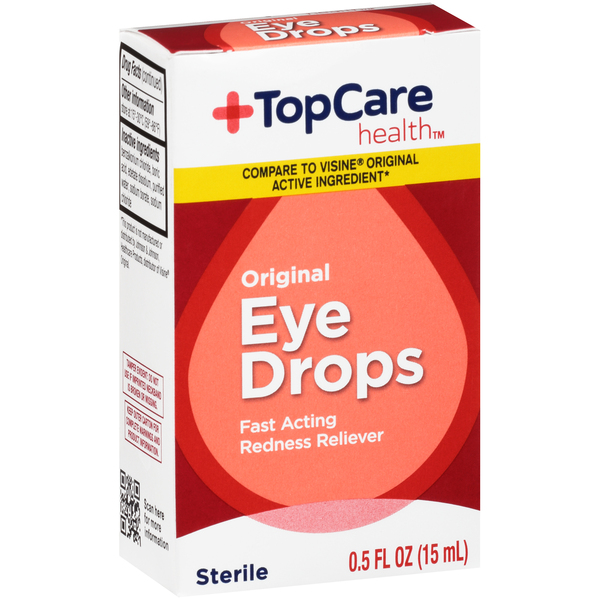
Which eyedrops are recalled and why are they dangerous?, Here's a full list of eye drops recalled in 2025: What eye drops have been recalled?

Recalled Eye Drops Linked to Infections, Vision Loss, Lubricant eye drops 15 ml (single pack) carboxymethylcellulose sodium 0.5%. Recalled products will have expiration dates ranging from february 2025 to september 2025.
:max_bytes(150000):strip_icc()/EzricareVerywell-8b7e959890a54f58905541916cc73bf6.png)
2 More Eye Drop Brands Recalled Due to Safety Risks, The manufacturer of eye drops sold under cvs health corp. Lubricant eye drops 15 ml (twin pack).
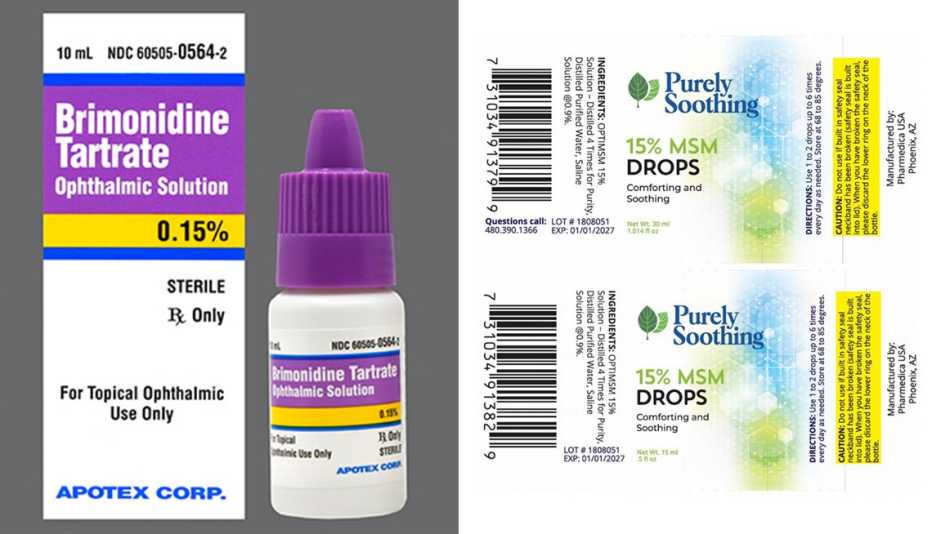
Eye drops recalled after 55 reports of bacterial infection, 1 death in, This new eye drop recall follows recalls earlier this year. Lubricant eye drops 15 ml.
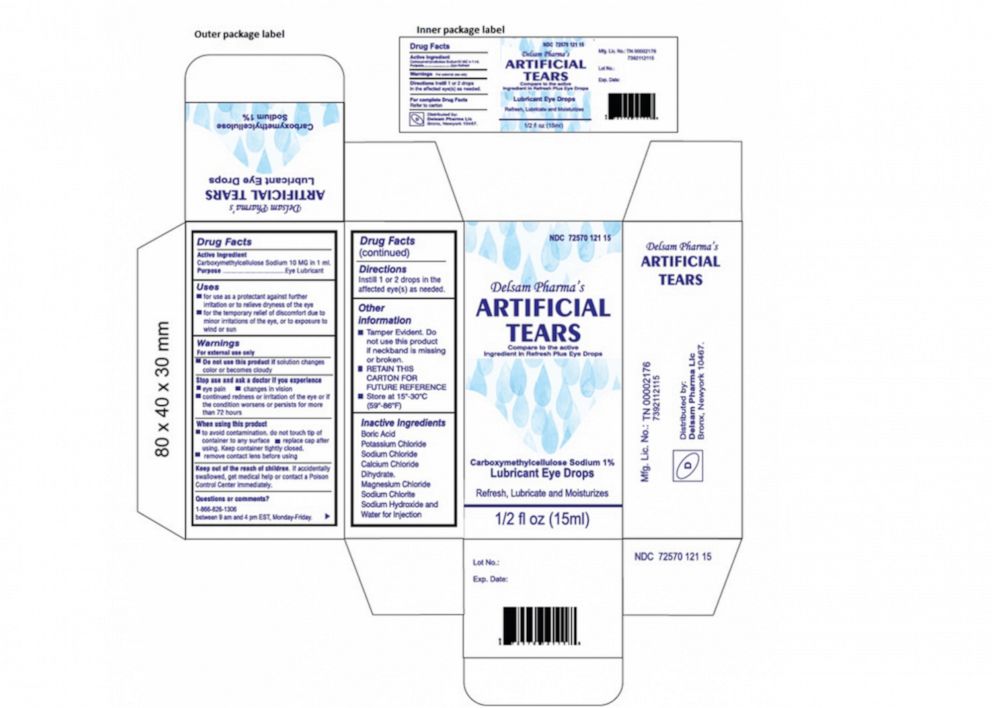
Recalled eye drops linked to multiple deaths and blindness across US, (wmt) and other brands has. The fda has expanded its list of eye drops recalled in 2025 because the products could be tainted with bacteria.

Eye Drops Recall 2025 Reasons, brand, and all you need to know, This new eye drop recall follows recalls earlier this year. Recalled products will have expiration dates ranging from february 2025 to september 2025.

Eye drops recalls Why are so many eye drops being recalled? Amazon, The recall followed a warning. Lubricant eye drops 15 ml (single pack) carboxymethylcellulose sodium 0.5%.

Eye Drops Recall 2025 List Every Brand Recalled Over, 49 OFF, The first eye drop recall involved artificial tears lubricant eye drops, which is manufactured by global pharma. This new eye drop recall follows recalls earlier this year.
